NIEF is a core research facility that serves the academia and industry of Puerto Rico, Latin American, and the Caribbean by providing advanced technology and training in confocal microscopy and electrophysiology.
Services
Fluorescence Microscopy
This technology makes possible the acquisition of fluorescence-based images of cellular structures and molecular events from biological samples and nanomaterials. For example, cellular organelle structures and neuronal action potentials, as well as intracellular calcium oscillations and drug delivery of nanoparticles, can be visualized with high temporal and spatial resolution.
Patch Clamp Electrophysiology
It is a versatile instrument to measure membrane action potentials and ion channel currents as well as the activity of single ion channels from any isolated cells or cellular tissues.
Imaging and Electrophysiology
The coupling of confocal microscopy with patch-clamp electrophysiology enables simultaneous acquisition of fluorescence images and bioelectrical signals from biological cells and tissues.
Applications
NIEF’s technologies can be used in a series of applications, for example:
- In vivo ultra-deep and high-speed imaging. For example, biochemical reactions, dynamics of biological processes, and cell interactions in deep areas within living organisms can be visualized at great speed and resolution.
- Simultaneous photostimulation and image acquisition at high-speed can be realized such as photoactivation, photoconversion, FRAP, FLIP, and photo caged-compounds.
- Simultaneous IR excitation imaging. Two different probes can be simultaneous excited with IR light and visualized.
- Digital videos of many important cellular functions such as muscle contraction, cell motility, cell division, and cytokinesis.
- Nanomedicine research. The drug delivery of therapeutic nanomaterials into cells can be visually monitored.
- Ion channel research. Ionic currents from ligand- and voltage-gated ion channels can be recorded with high fidelity as well as optical action potentials from any excitable cells.
- Neuroscience research. The structure and function of neuronal circuits in rats can be simultaneous analyzed for example the action potential firing of multiple neurons in a brain tissue.
Types of Samples
NIEF’s equipment can be used in:
- Primary & recombinant cells (e.g. neurons, cardiomyocytes, yeasts, bacteria, HEK, CHO)
- Cellular tissues (e.g. brain and heart slices, diaphragm, nephron, pancreas)
- Small animal models (e.g. zebrafish, mouse, rat, guinea pig)
- Nanomaterials (e.g. carbon fiber, nanoparticle)
Available Techniques
- Epi-Fluorescence Microscopy: Commonly known as widefield microscopy. Emission light excite the whole sample at the same time and digital pictures of the emission light may be capture with a Charge-Couple Device (CCD Camera) or a complementary metal oxide semiconductor (cMOS Camera). This technique maximizes the intensity of sample illumination.
- Confocal Laser Scanning Microscopy: Excitation laser reaches sample over the scanned area. Uses a spatial pinhole to block out-of-focus light that provides the ability to create optical sections of the sample. Axial resolution allows for a 3D reconstruction. Increases signal to noise ratio, reduce phototoxicity compare to Epi-fluorescence but only captures about 5% of all the emitted light.
- Spinning Disc Super Resolution Confocal Microscope: This technique combines the ability of blocking out-of-focus light from confocal with the high sensitivity of the Epi-fluorescence. Excitation is performed just as in Epi-fluorescence but collection of emitted light is made through a pinhole. Ideal for live cell imaging. Spatial resolution is improved to 120nm by deconvolution.
- Total Internal Reflection Fluorescence Microscope: Commonly known as TIRF. This technique restricts excitation of the sample to a thin region, typically near the coverslip (up to 200nm). Improves signal to noise ratio and therefore resolution. Ideal for studies in cell membrane and single molecule.
- Multiphoton Microscope: Also referred as Two-Photon Microscopy. Excitation comes from a tuning laser, wavelengths range from 700nm to 1000nm. Allows for deep tissue penetration and optical sectioning. Pototoxicity is reduce since excitation only occurs on focal plane. Ideal for whole brain samples, thick tissue slides, whole animal and development.
Service Fees
| Fee for services related to NIEF instruments | ||
|---|---|---|
| Instrument | UPR Investigator | *Non-UPR Investigator |
| Confocal Imaging | $75/hr | $100/hr |
| Two-photon Confocal | $75/hr | $100/hr |
| Super Resolution/TIRF | $125/hr | $200/hr |
| Electrophysiological Rigs | $50/6 hrs | $60/6 hrs |
| Spinning Disk | $75/6 hrs | $100/ 6 hrs |
| Technical Service for training in Microscopy | $100 / 4 hrs | $150 / 4 hrs |
| Technical Service for training in Electrophysiology | $200 / 8 hrs | $300 / 8 hrs |
| Technical Service to acquire the data in Microscopy | $75 / 4 hrs | $150 / 4 hrs |
| Technical Service to acquire the data in Electrophysiology | $75 / 4 hrs | $150 / 4 hrs |
*Investigators from academic institutions non-affiliated to the UPR may receive a discount, based on funding.
IMAGING
Confocal Microscopes
Nikon Eclipse Ti-E Inverted Fluorescence Microscope (for fixed cells, live-cell imaging & high-throughput imaging)
- A1R laser scanning confocal system
- LU-N4/N4S 4-laser unit (405 nm, 488 nm, 561 nm, 640 nm)
- Ultrafast resonant scanner (Up to 512 x 32 pixels at 420 fps)
- High-resolution galvano scanner (Up to 4096 x 4096 pixels)
- A1-DUG-2 GaAsP confocal multi-detector unit
- A1-DUS spectral detector unit (Up to 32-channel spectral image at 24 fps)
- Perfect focus system for live-cell imaging
- Lumencor SPECTRA X independent light engine (395, 440, 470, 510, 550, 575, 640 nm)
- Andor Zyla 4.2 PLUS sCMOS camera
- Tokai INU microscope incubator with temperature controller for top, stage, bath and lens and in-line 5% CO2 (for live-cell imaging)
- NIS Elements – High Content Analysis (HC) software








Nikon Eclipse FN1 Upright Fluorescence Microscope (for optogenetics, brain slices & tissue)
- A1R multiphoton laser scanning confocal system
- Coherent Chameleon II Vision multiphoton laser unit
- Episcopic GaAsP NDD multiphoton detector unit
- A1-DUS spectral detector unit
- LU-N4/N4 4-laser unit (405 nm, 488 nm, 561 nm, 640 nm)
- Ultrafast resonant scanner (Up to 512 x 32 pixels at 420 fps)
- High-resolution galvano scanner (Up to 4096 x 4096 pixels)
- A1-DUG-2 GaAsP confocal multi-detector unit
- High-speed piezo objective-positioning system
- DS-Fi1c high definition cooled color digital camera
- Lumencor SOLA SE white light engine
- CFI 75 Apochromat multiphoton objective with 25X, water immersion, NA 1.10, WD 2.00 mm, correction ring, and anti-reflective coating
- NIS Elements – Confocal (C) software
Nikon Eclipse FN1 Upright Fluorescence Microscope (for optogenetics, brain slices & tissue)
- A1R multiphoton laser scanning confocal system
- Coherent Chameleon II Vision multiphoton laser unit
- Episcopic GaAsP NDD multiphoton detector unit
- A1-DUS spectral detector unit
- LU-N4/N4 4-laser unit (405 nm, 488 nm, 561 nm, 640 nm)
- Ultrafast resonant scanner (Up to 512 x 32 pixels at 420 fps)
- High-resolution galvano scanner (Up to 4096 x 4096 pixels)
- A1-DUG-2 GaAsP confocal multi-detector unit
- High-speed piezo objective-positioning system
- DS-Fi1c high definition cooled color digital camera
- Lumencor SOLA SE white light engine
- CFI 75 Apochromat multiphoton objective with 25X, water immersion, NA 1.10, WD 2.00 mm, correction ring, and anti-reflective coating
- NIS Elements – Confocal (C) software




Nikon Eclipse Ti-E Inverted Fluorescence Microscope (at UPR-Institute of Neurobiology)
- A1R laser scanning confocal system
- LU-N4/N4S 4-laser unit (405 nm, 488 nm, 561 nm, 640 nm)
- Ultrafast resonant scanner (Up to 512 x 32 pixels at 420 fps)
- High-resolution galvano scanner (Up to 4096 x 4096 pixels)
- A1-DUG-2 GaAsP multi-detector unit
- A1-DUS spectral detector unit (Up to 32-channel spectral image at 24 fps)
- Perfect focus system for live-cell imaging
- Lumencor SOLA SE white light engine (395 nm, 420-680 nm)
- NIS Elements – Confocal (C) software




WORKSTATION COMPUTERS (for imaging analysis)
HP Z8 G4 workstation computer
- Operating System: Windows 10 Pro 64-bit English
- Chassis: HP Z8 G4 90 1125W Chassis 100V/15A
- Processor: 2X Intel® Xeon® Gold 5122 Processor (3.6 GHz, up to 3.7 GHz w/Turbo Boost, 16.5 MB cache, 2666MHz, 4 core), (8 Total Cores)
- Memory: 128 GB (8×16 GB) DDR4-2666 ECC Registered Memory (2 Processors)
- Internal OS Load Storage Options: Operating System Load to M.2
- Internal M.2 Storage: 512 GB HP Z Turbo Drive TLC M.2 SSD
- 2nd Internal M.2 Storage: 1 TB HP Z Turbo Drive TLC M.2 2nd SSD
- Internal Storage: 4X 2 TB 7200 RPM SATA 3.5” HDD, (8 TB Total)
- Graphic Card: NVIDIA® Quadro® P4000 (8 GB GDDR5, 4X Displayport 1.4) Graphics
- Networking: Intel® X550-T2 10GbE Dual Port NIC
- Ports: Premium – 2X USB 3.1 Type C; 2 x USB 3.0 Type A
- Keyboard: USB Premium Wired Keyboard
- Mouse: HP Wired Optical USB Mouse
- HP 24” Z24N G2 monitor
- Nikon NIS Elements – Advance Research (AR) software
- Nikon NIS Elements – Extended Depth of Focus (EDF) module
- Nikon NIS Elements – Calcium Ratio and FRET module
- Nikon NIS Elements – General Analysis in 2D and 3D module
- Nikon NIS Elements – 2D and 3D Measurement Tracking module
- Nikon NIS Elements – Laboratory Imaging (LIM) 2D and 3D Deconvolution module
- Nikon NIS Elements – Additional Batch Offline LIM Deconvolution module
HP Z8 G4 workstation computer (at UPR-Institute of Neurobiology)
- Operating System: Windows 10 Pro 64-bit English
- Chassis: HP Z8 G4 90 1125W Chassis 100V/15A
- Processor: 2X Intel® Xeon® Gold 5122 Processor (3.6 GHz, up to 3.7 GHz w/Turbo Boost, 16.5 MB cache, 2666MHz, 4 core), (8 Total Cores)
- Memory: 128 GB (8×16 GB) DDR4-2666 ECC Registered Memory (2 Processors)
- Internal OS Load Storage Options: Operating System Load to M.2
- Internal M.2 Storage: 512 GB HP Z Turbo Drive TLC M.2 SSD
- 2nd Internal M.2 Storage: 1 TB HP Z Turbo Drive TLC M.2 2nd SSD
- Internal Storage: 4X 2 TB 7200 RPM SATA 3.5” HDD, (8 TB Total)
- Graphic Card: NVIDIA® Quadro® P4000 (8 GB GDDR5, 4X Displayport 1.4) Graphics
- Networking: Intel® X550-T2 10GbE Dual Port NIC
- Ports: Premium – 2X USB 3.1 Type C; 2 x USB 3.0 Type A
- Keyboard: USB Premium Wired Keyboard
- Mouse: HP Wired Optical USB Mouse
- HP 24” Z24N G2 monitor
- Nikon NIS Elements – Advance Research (AR) software
- Nikon NIS Elements – Extended Depth of Focus (EDF) module
- Nikon NIS Elements – Calcium Ratio and FRET module
- Nikon NIS Elements – General Analysis in 2D and 3D module
- Nikon NIS Elements – 2D and 3D Measurement Tracking module
- Nikon NIS Elements – Laboratory Imaging (LIM) 2D and 3D Deconvolution module
- Nikon NIS Elements – Additional Batch Offline LIM Deconvolution module
DELL XPS 15 Laptop (2 laptops)
- Operating System: Windows 10 Pro 64-bit English
- Processor: 8th Generation Intel® Core™ i7-8750H Processor (9MB Cache, up to 4.1 GHz, 6 Cores)
- Memory: 32GB 2x16GB DDR4-2666MHz,
- Hard Drive: 1TB PCIe Solid State Drive
- Video Card: NVIDIA® GeForce® GTX 1050Ti with 4GB GDDR5 graphics
- Display: 15.6″ FHD 1920 x 1080 Anti-Glare Non-touch IPS 100% sRGB 400-Nits display InfinityEdge
- Wireless: Killer 1535 802.11ac 2×2 WiFi and Bluetooth 4.2
- Software: (TBA)
DELL XPS 15 Laptop (2 laptops at UPR-Institute of Neurobiology)
- Operating System: Windows 10 Pro 64-bit English
- Processor: 8th Generation Intel® Core™ i7-8750H Processor (9MB Cache, up to 4.1 GHz, 6 Cores)
- Memory: 32GB 2x16GB DDR4-2666MHz,
- Hard Drive: 1TB PCIe Solid State Drive
- Video Card: NVIDIA® GeForce® GTX 1050Ti with 4GB GDDR5 graphics
- Display: 15.6″ FHD 1920 x 1080 Anti-Glare Non-touch IPS 100% sRGB 400-Nits display InfinityEdge
- Wireless: Killer 1535 802.11ac 2×2 WiFi and Bluetooth 4.2
- Software: (TBA)
ELECTROPHYSIOLOGY
ELECTROPHYSIOLOGY RIGS
Patch Clamp Rig (for brain slices and tissue)
- Coupled to Nikon A1R multiphoton FN1 upright fluorescence microscope
- Axopatch 200B amplifier
- Axon Digidata 1550B digitizer
- P.I. Master-9 pulse stimulator
- Sutter MPC-200 multi-micromanipulator system
- Axon pCLAMP 10 software




Patch Clamp Rig (for isolated cells & cell lines)
- Coupled to Nikon Ti2-A inverted fluorescence microscope
- HEKA 10 USB amplifier
- Andor Zyla 4.2 PLUS sCMOS camera
- Thorlabs PCS-5200 micromanipulator
- Warner TC-344C temperature controller
- Warner SH-27B in-line solution heater
- Warner QE-1 quick exchange chamber platform
- Lumencor SOLA SE white light engine (395 nm, 420-680 nm)
- HP Z4 G4 workstation computer
- HP 24” Z24N G2 monitor
- HEKA PatchMaster software
- Andor iQ software
Patch Clamp Rig (for isolated cells & cell lines)
- Coupled to Nikon Ti2-A inverted fluorescence microscope
- HEKA 10 USB amplifier
- Andor Zyla 4.2 PLUS sCMOS camera
- Thorlabs PCS-5200 micromanipulator
- Warner TC-344C temperature controller
- Warner SH-27B in-line solution heater
- Warner QE-1 quick exchange chamber platform
- Lumencor SOLA SE white light engine (395 nm, 420-680 nm)
- HP Z4 G4 workstation computer
- HP 24” Z24N G2 monitor
- HEKA PatchMaster software
- Andor iQ software


Patch Clamp Rig (for isolated cells & cell lines)
- Coupled to Olympus IX70 inverted fluorescence microscope
- Axopatch 200B amplifier
- Axon Digidata 1440A digitizer
- Sutter MP-285 micromanipulator
- Warner VC-6 valve controller
- Warner SF-77B fast stepper perfusion system
- Warner TC-344C temperature controller
- Warner SH-27B in-line solution heater
- Warner QE-1 quick exchange chamber platform
- Olympus BH2-RFL-T3 mercury arc lamp with 100 W bulb (lEx: 254, 297-302, 312-313, 334, 365, 405, 436, 546, and 579 nm)
- Andor iXon EMCCD camera
- Axon pCLAMP 10 software
- Andor iQ software



Sharp Electrodes Intracellular Recording Rig (for tissue)
- Coupled to Olympus BX51WI upright fluorescence microscope
- Axoclamp 900A amplifier
- Axon Digidata 1440A digitizer
- Sutter MPC-200 multi-micromanipulator system
- Sutter ROE-200 micromanipulator controller
- Axon pCLAMP 10 software
Sharp Electrodes Intracellular Recording Rig (for tissue)
- Coupled to Olympus BX51WI upright fluorescence microscope
- Axoclamp 900A amplifier
- Axon Digidata 1440A digitizer
- Sutter MPC-200 multi-micromanipulator system
- Sutter ROE-200 micromanipulator controller
- Axon pCLAMP 10 software

Two-Electrode Voltage Clamp Rig (for frog oocytes)
- GeneClamp 500 amplifier
- Axon Digidata 1440A digitizer
- Warner VC-8 valve controller
- Axon pCLAMP 10 software




Two-Electrode Voltage Clamp Rig (for frog oocytes)
- GeneClamp 500B amplifier
- Axon Digidata 1440A digitizer
- Warner VC-8 valve controller
- Axon pCLAMP 10 software
BILAYER STATION

Planar Lipid Bilayer Workstation
- Warner BC-535 bilayer clamp amplifier
- Warner FC-1 Faraday cage with manual vibration isolation table
- Warner LPF-8 Low-pass Bessel filter
- Warner BPS-2 Bilayer Perfusion system
- Warner SUNStir-3 Dual function controller for lamp stirplate
- Axon pCLAMP 10 software
ELECTROMYOGRAPHY (EMG) STATION
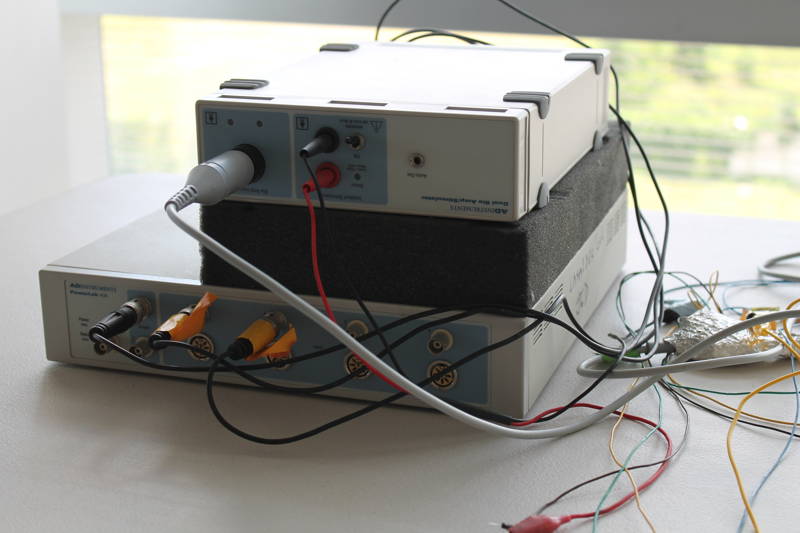
Electromyography Workstation (for small animals)
- AD PowerLab 4/30 system
- AD ML408 Dual Bio amplifier and stimulator
- AD LabChart 6 software
TRAINING
NIEF provides training opportunities for graduate students, postdoctoral fellows, young investigators, and faculty on imaging and electrophysiology techniques.